abbott rapid covid test how to use
Furthermore the two versions of the Abbott antigen. 50 out of 5 stars 2.

Panbio Covid 19 Ag Rapid Test Device Diagnostic Rapide En Poc Abbott
Panbio COVID-19 Ag Rapid Test Device Nasopharyngeal with 2D barcode 25T.
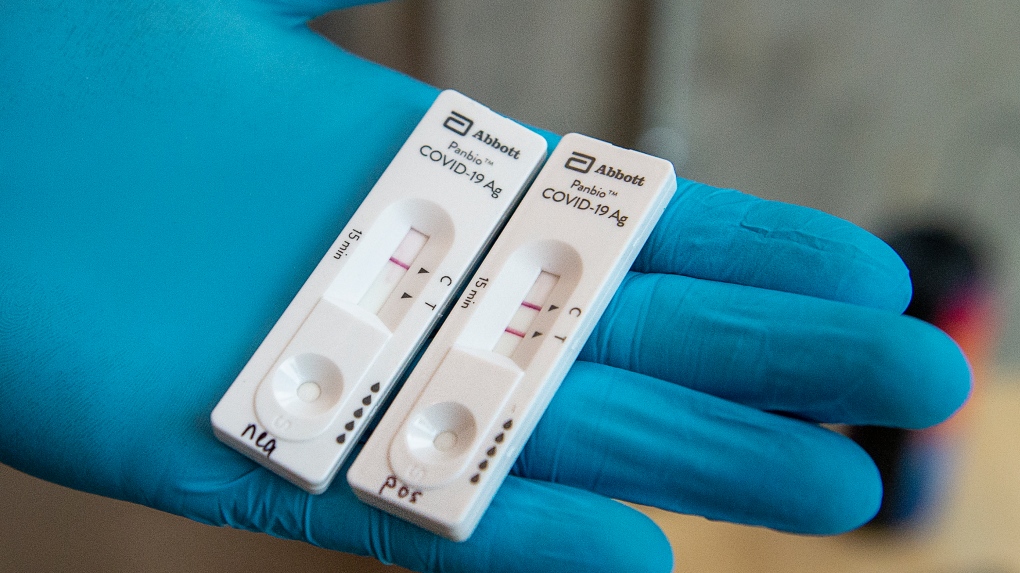
. This is typically a highly. The rapid test detects COVID-19 caused by different variants. It should be noted that while Abbott already markets an antigen rapid test that is in widespread use in the United States BinaxNOW COVID-19 Antigen Card the antigen test which has been approved for use and is being marketed in Canada Panbio COVID-19 Antigen Rapid Test is manufactured in a different facility. ABT announced today that the US.
Next you place the swab in. Like Abbotts BinaxNOW test the Quidel antigen test also works like a pregnancy test. The test provides preliminary test. First you take a nasal swab then mix the swab with a tube of liquid for one minute.
ABBOTT PARK Ill Dec. Simply test yourself twice within 3 days with at least 36 hours between. A simple solution for COVID-19 infection detection this 15-minute test can be completed anytime anywhere. The Abbott ID Now test utilizes a nucleic acid molecular detection method similar to their influenza A and B tests.
Lucira Check It COVID-19 Test Kit The Only FDA EUA Single-use PCR Quality Molecular Test Easy to use Results at Home in 30. This rapid-result test is for personal use. Quidel QuickVue rapid test. Abbott will sell this test for 5It is highly portable about the size of a credit card affordable.
Negative results dont preclude. COVID -19 Ag CARD. Shortly after Abbott completed its delivery of 150 million rapid antigen tests to the federal government researchers at the CDC said the card-based diagnostic may fail to catch about two-thirds. COVID-19 antigen rapid test ART kits for self-testing will be dispensed in pharmacies to the public from Wednesday Jun 16 the Ministry of Health MOH announced on Jun 10.
This website is governed by applicable US. The Abbott ID Now Rapid Molecular Test for COVID-19 is the first in-house lab testing available to MemorialCare. The minimum order quantity is 25 tests 1 box. COVID-19 Rapid Antigen Test Panbio.
The portable rapid molecular ID NOW COVID-19 test has emerged as a critical part of this arsenal allowing fast diagnosis with results in 13 minutes or less in a variety of locations such as physicians offices urgent care clinics and other point-of-care locations. Abbott is bringing BinaxNOW the most affordable and widely. Laws and governmental regulations. No use of any Abbott trademark trade name or trade dress in this site may be made without the prior written authorization of Abbott except to identify the product or services of the company.
The product may be used in any laboratory and non-laboratory environment that meets the requirements specified in the Instructions for Use and local regulation. Food and Drug Administration FDA has issued Emergency Use Authorization EUA for its BinaxNOW COVID-19 Ag Card rapid test for detection of COVID-19 infection. If youre interested in rapid testing solutions contact us today for a quoteYou can also place an order for the Abbott Panbio COVID-19 Rapid Antigen Test Device nasal or other rapid tests approved by Health Canada through our online rapid test shop. Find out more about this innovative technology and its impact here.
It has been available within MemorialCare for about two weeks and there remains some confusion about its proper use in testing. Ellumes rapid COVID-19 test can send results to your smartphone in 15 minutes. The product may be used in any laboratory and non-laboratory environment that meets the requirements specified in the Instructions for Use and local regulation. Leave the card sealed in its foil pouch until just before use and do not use the card if the card or foil pouch is open damaged expired or if the blue line is not present.
Ohio has invested in rapid COVID-19 testing with a goal of using fast free and convenient testing to control the spread of COVID-19. Our team at Rapid Test Trace Canada can also assist with implementing a. COVID-19 Ag Card Test Helpful Testing Tips continued During the Test. ABT announced today that the US.
This test is authorized for use with direct anterior nasal nares swab samples from individuals without symptoms or other epidemiological reasons to suspect COVID-19 when tested twice over three days with at least 36 hours between tests. 2 Panbio COVID-19 RAPID TEST DEVICE Nasal Abbott Rapid Diagnostic Jena GmBH Jena Germany Antigen Nasal 3 Biocan Tellmefast Covid-19 Antigen Test Biocan Diagnostics Inc Canada Antigen Saliva 4 INNOVITA One Step Rapid Test 2019-nCoV Ag Test Latex Chromatography Assay INNOVITA TANGSHAN BIOLOGICAL TECHNOLOGY CO. While Abbott advertises a sensitivity of 917 which would mean that out of 1000 people who have COVID-19 the test would correctly identify 917 of them another study found that test sensitivity was only 74 and for self-collected samples even lower. IHealth COVID-19 Antigen Rapid Test 90 Packs2 Tests per PackFDA EUA Authorized OTC at-Home Self Test Results in 15 Minutes with Non-invasive Nasal Swab Easy to Use No Discomfort.
Get back to business and life thanks to Abbott BinaxNow COVID-19 Tests. BinaxNOW COVID-19 Antigen Home Rapid Self-Testing Kit by Abbott 2 Tests 3895 2495-36 New. 1 offer from 149900. COVID-19 rapid antigen tests also frequently called COVID-19 lateral flow tests or LFTs are rapid antigen tests used to detect SARS-COV-2 infection They are quick to implement with minimal training and offer significant cost advantages costing a fraction of other forms of COVID-19 testing and give users a result within 530 minutes.
Fortunately specificity the likelihood of getting a false positive is more accurate 985-100 so out of. The state is working with local health departments. Testing is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 CLIA. The test provides preliminary test results.
ABBOTT PARK Ill Aug. 15 SAMPLE CONTROL. 26 2020 PRNewswire -- Abbott NYSE. Below are details about the various partners we are working with to make testing available as well as information for employers who are interested in initiating testing programs.
Panbio COVID-19 Ag Rapid Test Device is for professional use only and is intended to be used as an aid in the diagnosis of SARS-CoV-2 infection. The app then reports the results to public health experts. Abbott BinaxNOW COVID-19 Test Kits in Bulk. The box includes 2 tests that are indicated for serial testingtest yourself.
Abbott conducted a computational analysis of the detection of multiple SARS-COV-2 Strains including the Delta variant and predicts no impact to the performance of our BinaxNOW COVID-19 Antigen Self Test. Panbio COVID-19 Ag Rapid Test Device is for professional use only and is intended to be used as an aid in the diagnosis of SARS-CoV-2 infection. BinaxNOW COVID-19 Self-Test at Home Kit offers rapid results in the convenience of your home. Rapid antigen tests such as the Abbott BinaxNOW COVID-19 Ag Card BinaxNOW offer results more rapidly approximately 1530 minutes and at a lower cost than do highly sensitive nucleic acid amplification tests NAATs 1Despite a lower sensitivity to detect infection rapid antigen tests can be an important tool for screening because of their quick.
Not everyone is vaccinated yet so these trusted and easy-to-use tests can detect COVID-19 in both symptomatic and asymptomatic people providing added peace of. Learn how to use the BinaxNOW COVID-19 Antigen Self Test and store your results in the NAVICA app for self testing. See results in just 15 minutes. Food and Drug Administration FDA has issued Emergency Use Authorization EUA for virtually guided at-home use of its BinaxNOW COVID-19 Ag Card rapid test for detection of COVID-19 infection.
Correct x6 Wrong. BinaxNOW COVID-19 Antigen Self Test from Abbott can be used to detect active infection with or without symptoms. 16 2020 PRNewswire -- Abbott NYSE. Abbott PANBIO COVID-19 Ag Rapid Test device is an In Vitro Diagnostic Rapid Antigen Test for qualitative detection of SARS-CoV-2 Antigen Ag directed for Healthcare ProfessionalsAbbott Panbio COVID-19 Rapid Antigen Test device can identify potentially COVID-19 contagious patients with or without symptoms in 15 minutes to reduce.

How To Use Rapid Testing To Keep You And Yours Safe During The Holiday Season Victoria News

17 Long Term Care Homes In Ottawa Implement Rapid Covid 19 Testing Ctv News

Abbott Labs Rolls Out Rapid Covid Test To Us Schools And Workplaces

White House Will Spend Another 1 Billion On Rapid At Home Covid Tests Shots Health News Npr

Panbio Covid 19 Ag Rapid Test Device Diagnostic Rapide En Poc Abbott
Posting Komentar untuk "abbott rapid covid test how to use"